Chemical Reactions of aldehydes and Ketones, Distinction between .aldehydes and ketones
Table of Contents:
1
Introduction
1.1
What are aldehydes?
1.2
What are ketones?
2
Chemical Reactions
2.1
Nucleophilic addition reactions.
2.1.1
General Reaction
2.1.2
Ways in which
Reaction occurs
2.2
Examples of Nucleophilic Addition in Aldehydes and
Ketones
2.2.1
Attack of cyanide.
2.2.2
Condensation
Reaction
2.2.3
Canizzaro’s
Reaction
2.3
Reduction Reaction
2.4
Oxidation reaction
3
Reversibility of Nucleophilic Addition
4
Relative reactivity: Aldehydes vs Ketones
4.1
Stearic Factors
4.2
Electronic Factors
5
Practical distinctions between aldehydes and ketones
5.1
Tollen’s Test (Silver Mirror Test)
5.2
Fehling’s Solution/Benedict’s Solution test
5.3
Reaction with acidified Dichromate (4) Solution
5.4
Sodium Nitropuside test
5.5
·
Introduction:
Aldehydes:
These are theorganic compounds that have
a carbonyl group bonded to a carbon atom on one side while hydrogen atom is
present on the other side of the functional group. (Except for Formaldehyde
that has hydrogen atom on both sides of carbonyl group)
General Formula:
Ketones:
These are compounds having carbon atom
on both sides of the carbonyl carbon.
General Formula:
For example: Acetone
Chemical Reactions:
The most important reaction of ketones
and aldehydes is nucleophilic
addition to the C-O double bond of carbonyl group.
In general, the negative part of reagent
i.e. Nucleophile attaches to carbon atom while the positive part of reagent
(usually H) combines with Oxygen atom of carbonyl group.
As a result, addition product ‘adduct’
is obtained.
General Reaction:
For Example:
·
Generally,
additions to carbonyl groups consist of two mechanistic steps:
1. Attack
of a nucleophile on carbonyl group.
2. Protonation
of anion thus formed.
NUCLEOPHILIC ADDTION TO CARBONYL GROUP
Aldehydes and ketones undergo
nucleophilic addition because of their special structural features:
§ The
groups attached to carbonyl Carbon are arranged in trigonal planner symmetry
which means the Carbonyl carbon atom is openly available to be attacked from above
or below the plane of carbonyl group.
§ The
carbonyl carbon atom is positively charged which means it is especially
susceptible to be attacked by a nucleophile.
§ The
carbonyl oxygen is negatively charged that means, nucleophilic addition is susceptible
to acid catalysis.
Occurrence of the reaction:
Nucleophilic addition to C-O double bond
occurs in one of the two general ways;
1.
When
the reagent is a strong nucleophile: The trigonal planer
structure of aldehyde or ketone is converted into the tetrahedral product as a
result of the addition. In this type of reaction, the Nu uses its electron pair
to form bond to the carbon atom of carbonyl group. As it happens, the electron
pair of C-O pi bond shifts to electronegative oxygen of carbonyl group and
hybridization of carbon and oxygen atoms shifts from sp2 to sp3. The important thing about this step is that
oxygen atom of carbonyl group is capable of accommodating electron pair of C-O
double bond.
In
the second step, oxygen receives a proton. This happens due to full negative
charge of oxygen atom and it’s much more basic nature.
2.
When
the nucleophile is weak and an acid catalyst is present: The
electrophilicity of carbonyl group is increased by the reaction of oxygen atom of
carbonyl group with the acid.
The mechanism operates when the carbonyl
compounds react with strong acidsin the presenceof weak
electrolytes. Acid donates a proton to the oxygen of carbonyl group in
the first step. An oxoniumcation, a protonated carbonyl compound, thus
formed is highly reactive towards nucleophic attack on the carbon atom of
carbonyl group because as compared to unprotonated compound, it have more
positive charge.
EXAMPLES OF NUCLEOPHILIC ADDITION
·
Attack
of a Cyanide:
Cyanohydrins
are obtained as hydrogen cyanide adds to the aldehydes and ketones. The
reaction is carried out by slow addition of a mineral acid to an aqueous
solution of sodium cyanide. The acid generates the hydrogen cyanide from sodium
cyanide.
H3C HCl CH3
CN
Acetaldehyde
AcetaldehydeCyanohidrin
H3C
H3C CN
Acetone
Acetone cyanohydrin
Mechanism
of Reaction:
HCN does not have any lone pair.
Base (OH-) produces the cyanide ion as a nucleophile.
At
the end of the reaction the hydroxyl ion is re-produced. These OH- ions reacts
with more and more HCN molecules to generate more CN- ions. In this way, the
reaction continuous.
·
Condensation
Reaction:
The
reaction where two molecules of similar or different compounds combine to give
a new compound where the elimination of a small molecule, like water or
ammonia, may or may not takes place is called as a condensation reaction.
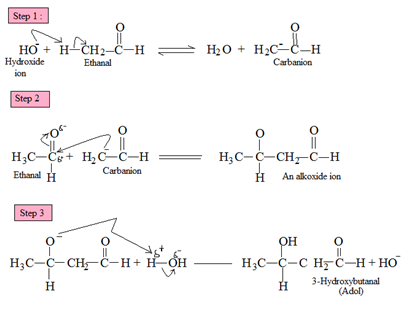
Condensation
reaction of two carbonyl compounds to generate an adol product is called
as an adol condensation reaction.
Aldehydes
and ketones that have alpha-hydrogen atom react with a cold dilute alkali
solution to produce addition products that are known as aldols.
dilNaOH
CH3 propanone(acetone) CH3
Propanone(Acetone)
4-hydroxy-4-methyl-2-pentanone
Mechanism of the Reaction:
a. The
hydroxide ion acts as a base. It removes a proton from alpha-carbon of one
molecule of the carbonyl compoundto form a carbanion.
b. The
caranion here acts as a nucleophile. It attacks the electrophilic carbonyl
carbon atom of the second molecule to form an alkoxide ion.
c. The
alkoxide ion removes a proton from water to form an aldol. The basic catalyst
hydroxide ion is produced.
·
Canizzaro’s
reaction:
In the presence of a base, the
disproportionation reaction(self-oxidation reduction process) of aldehydes that
contains no alpha-hydrogen is called a Canizzaro’s reaction.
Formaldehyde
Ethanol Sodium Formate
Mechanism of reaction:
I.
Attack of
Nucleophile: The hydroxide ion acts as a nucleophile. It attacks on the
electrophile carbonyl carbon atom to form a complex anion.
II.
Transfer of
ions: The anion transfers a hydride ion to second molecule of formaldehyde.
The
presence of negative charge on oxygen of the anion helps in the loss of hydride
ion.
III.
Formation of
Formate ion: The methoxide ion acts as a base and gets a proton from formic
acid to form methanol and formate ion.
The
formate ion in the presence of alkali gives a salt of the acid.
REDUCTION
REACTIONS
Both aldehydes and ketones can undergo
reduction process (addition of water). Aldehydes are reduced to primary
alcohols and ketones to secondary alcohols.
a)
Reduction
with sodium Borohydride:
Alcohols are formed when carbonyl
compounds react with sodium borohydride. The reaction occurs by addition of
sodium borohydride to an alcoholic or aqueous solution of aldehyde or ketone.
Mechanism
of reaction:
1) Sodium
borohydride gives tetrahydroborate (3) ion. The tetrahydroborate ion, is the
source of hydride ion.
2) This
hydride ion acts as a nucleophile that attacks on electrophilic carbon atom of
the carbonyl group to give an alkoxide ion.
3) The
alkoxide ion is protonated with water to give an alcohol.
b)
Catalytic
Reduction:
The
aldehydes and ketones when undergo reduction with hydrogen in the presence of a
metal catalyst like Pd, Pt or Ni from primary alcohols and secondary alcohols
respectively.
Hydrogen
is added across the carbonyl group.
For
Example:
OXIDATION
REACTIONS
i.
Oxidation
of Aldehydes:
Aldydes
undergo oxidation easily by mild oxidizing agent as Fehling’s reagent, Tollen’s
reagent and Banedict’ssolution.They can also be converted to carboxylic acids
by strong oxidizing agents like sulphuric acid/potassium dichromate and dilute
nitric acidThe aldehyde carbonyl group is converted to OH group. The carboxylic
acid thus obtains has the same number of C atoms as were present in aldehyde.
ii.
Oxidation
of Ketones:
Oxidation
of ketones do not occur easily because of the reason that they require strong
C-C bond breakage. With mild oxidizing agents, they do not give reaction.Only
strong oxidizing agents can oxidize the ketones like potassium dichromate\
sulphuric acid and conc. HNO3. During
oxidation process of ketones, only carbon atom that is adjacent to carbonyl
group is attacked. In general, the C atom having smaller number of hydrogen
atom is oxidized.
For
symmetric ketones, only one C atom present nearest to Carbonyl group is
oxidized and two carboxylic acids in a mixture are obtained.
However,
for unsymmetrical ketones, the C atom attached with smaller number of H
atoms is oxidized and carbonyl group remains attached with smaller alkyle
group.
REVERSIBILITY
OF NUCLEOPHILIC ADDTION REACTIONS
As many C-O double bond are reversible,
the overall reaction result depends upon the position of equilibrium. This is
in contrast to most of the nucleophilic addition to C-O double bonds and with
nucleophilic substitution at saturated c atoms.
REALTIVE REACTIVITY OF CARBONYL
COMPOUNDS:
ALDEHYDES VS KETONES
Generally aldehydes are more reactive in
nucleophilic addition reactions than the ketones. Steric factor and electronic
factor both favor aldehydes.
Steric Factor: In aldehydes, as one group is C atom, the
central C atom of the tetrahedral product thus formed from aldehyde contains
lesser groups and product becomes more stable. The
product formation is therefore favored at equilibrium. In ketones the two alkyl
groups attached to carbonyl C result in higher steric crowding in tetrahedral
product as a result the product becomes less stable. Hence, a smaller
amount\concentration of product is available on equilibrium.
Electronic factors: As
alkyl groups are electron releasing compounds,therefore, aldehydes are more
reactive than ketones on electronic ground. Aldehydes contain single electron
releasing group that partially neutralize and hence stabilize the positive
charge at the C atom of carbonyl group. Ketones, on the other hand, having two
carbonyl groups are more atable. In short, the equilibrium constant for the
formation of product that is a tetrahedral compound from a ketone is smaller
and hence, the reaction is unfavorable.
PRACTICAL DIFFERENCES BETWEEN ALDEHYDES
AND KETONES
The
difference between an aldehyde and a ketone is the presence of a hydrogen atom
attached to the Carbon-Oxygen double bond in aldehydes. Ketones don't have that
hydrogen.
The
presence of that hydrogen atom makes aldehydes very easy to oxidize. Because
ketones don't have that particular hydrogen atom, they are resistant to
oxidation, and only very strong oxidizing agents like potassium manganate (Vll)
solution oxidize ketones. However, they do it in a destructive way, breaking
carbon-carbon bonds. So, by avoiding using these powerful oxidizing agents, we
can easily differ between an aldehyde and a ketone. Aldehydes are easily
oxidized by all sorts of oxidizing agents while ketones are not.
Tollen’s Test
(Silver Mirror Test):
Tollen's
reagent contains the diamminsilver(l) ion. This is made from silver nitrate
solution. A drop of sodium hydroxide solution is added to a precipitate of
silver oxide, then enough dilute ammonia solution is added to redissolve the
precipitate. To carry out the test, A few drops of the aldehyde or ketone is
added to the freshly prepared reagent, and warmed gently in a hot water bath
for few minutes.
Ketone:
There is no change in colorless solution.
Aldehyde:
The colorless solution forms the grey precipitates of silver or a silver mirror
in the test tube.
Aldehydes
reduce the diamminsilver(l) ion to metallic silver. Because the solution would
be alkaline, the aldehyde itself will be oxidized to a salt of the
corresponding carboxylic acid. The electron-half-equation for the reduction of
the diamminsilver (l) ions to silver is:
Combining that with the
half-equation for the oxidation of an aldehyde under alkaline conditions:
Gives the overall equation:
Benedict’s Solution Test or Fehling’s
Solution Test:
Fehling's
solution and Benedict's solution are the variants of essentially the same
thing. Both contain complexed copper(ll) ions in an alkaline solution.
◼️
Fehling's solution contains copper(ll) io s complexed with tartrate iond in
sodium hydroxide solution.
Complexing
the copper(ll) ions with tartarate ions prevents the precipitation of
copper(ll) hydroxide.
◼️
Benedict's solution contains copper(ll) ions complexed with citrate ions in
sodium carbonate solution. Again, complexing the copper(ll) ions prevents the
precipitation formulation, this time of copper(ll) carbonate.
Both
solutions are used in the same way. A few drops of the aldehyde or ketone are
added to the reagent, and the mixture is warmed gently in a hot water bath for
a few minutes.
Ketone:No
change in blue solution.
Aldehyde:The
blue solution produces a dark red precipitate of copper(ll) oxide.
Aldehydes
reduce the complexed copper ll ion to copper l oxide. Because the solution is
alkaline, the aldehyde itself is oxidized to a salt of the corresponding
carboxylic acid. The equation for these reactions is always simplified to avoid
having to write in the formula for the tartarate or citrate ions in the copper
complexes. The electron-half-equations for both Fehling's solution and
Benedict's solution can be written as:
Combining
that with the half-equation for the oxidation of an aldehyde under alkaline
conditions:
Overall
equation becomes:
Using
acidified potassium dichromate solution:
A
small amount of potassium dichromate solution is acidified with dilute
sulphuric acid and a few drops of the aldehyde or ketone are added. If nothing
happens in cold the mixture is warmed gently for a couple of minutes- for
example, in a beaker of hot water.
Ketone:No
change in the orange solution.
Aldehyde:Orange
solution turns green.
The
orange dichromate ions have been reduced to green chromium ions by the
aldehyde. The electron-half-equation for the reduction of dichromate ions is:
Combining
that with the half-equation for the oxidation of an aldehyde under acidic
conditions:
Overall
reaction becomes:
Sodium
Nitropruside Test:
Ketones
produce an orange or wine red colour after adding alkaline sodium nitroprusside
solution drop by drop.
Aldehydes do not give this test.
CONCLUSION:
Ketones and aldehydes are important
carbonyl compounds, most important of their reactions is nucleophilic addition
to the C-O double bond. Aldehydes are more reactive than ketones and can be
distinguished through a variety of chemical tests.




















0 Comments